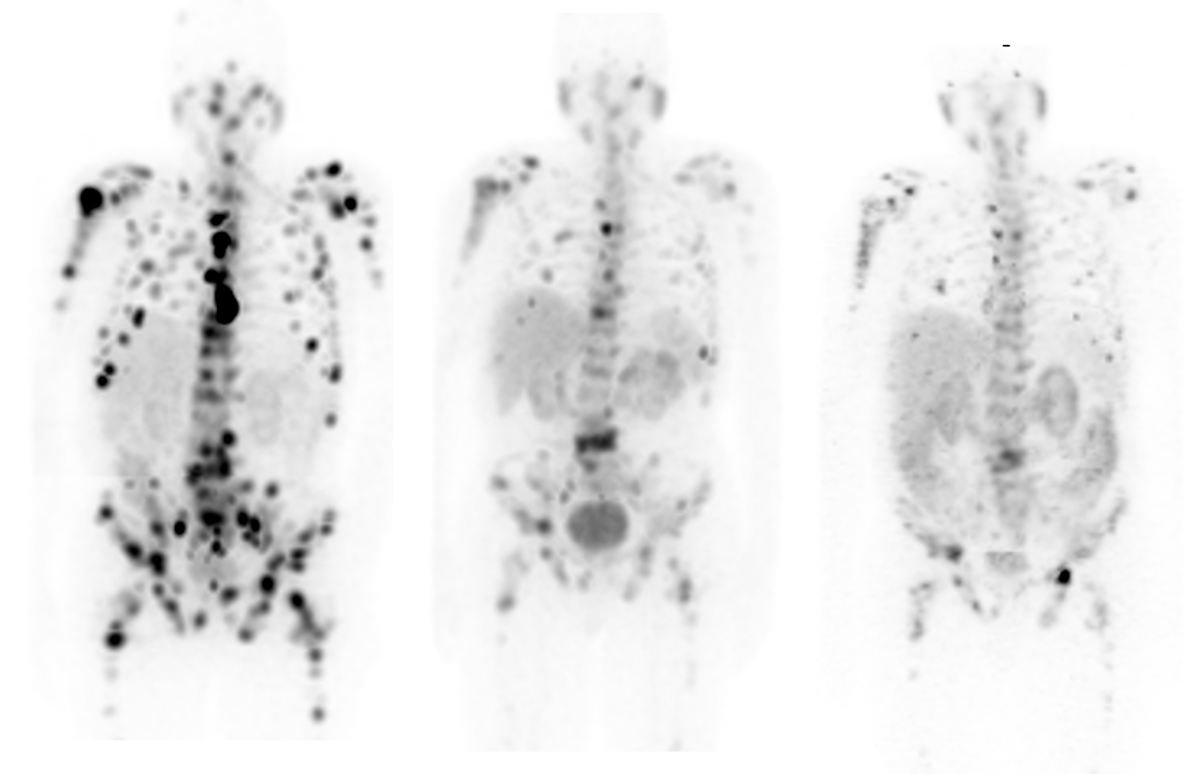
Theranostics is an exciting field of molecular medicine. Enabled by molecular imaging techniques such as positron emission tomography (PET) and single photon emission tomography (SPECT), the theranostic approach uses specific targeting compounds for both imaging and therapy of a particular malignancy. Its ability to identify areas of disease that are more likely to respond to targeted therapies is invaluable to cancer patients. And clinicians are praising its successes and its potential to help them more effectively navigate disease management by aligning patients with the treatments that will be most impactful for them.
With the evolution of advanced imaging technologies, and the continuous search to discover new tracers for targeted therapies, industry leaders such as GE Healthcare are fortifying the entire molecular imaging pathway, from providing access to emerging molecules to continuing to push the limits of molecular imaging with PET/computed tomography (CT) and SPECT/CT technologies. Innovations in molecular imaging technology introduce much more imaging data for processing and include highly sophisticated automated tools and artificial intelligence (AI)-based reconstruction algorithms to assist clinicians as they render complex diagnoses. Molecular imaging is essential in theranostics, allowing for non-invasive, repetitive assessment of the compound uptake and allowing for characterisation of the tumor tissue, and therapy response over time.
In this data-rich environment, theranostic target pairs have been developed, validated, and successfully used in treating lymphomas, neuroblastoma, neuroendocrine tumors and more recently, certain prostate cancers.[1] Strong clinical need in areas such as prostate and other cancers continue to fuel the search for additional diagnostic and therapeutic pairings with the goal of improving quality of life and outcomes for cancer patients.
Success with theranostics in prostate cancer
When clinically relevant prostate cancer is found and treated at an early stage before metastasis has occurred, treatments such as prostate cancer surgery and radiation often result in improved survival.[2] Worldwide, however, prostate cancer is the most commonly diagnosed male malignancy and the fourth leading cause of cancer death in men.[3] Current screening methodologies for prostate cancer include blood test to quantify prostate-specific antigen (PSA), or hormone levels and common treatments include radical prostatectomy combined with radiation therapy, however, this route is not always a possibility due in part to the complex process required to detect tumors.1
Despite advances in treating prostate cancer, certain prostate cancer types, called castrate- or hormone-resistant, continue to grow even when the patients’ hormone levels reach beyond the established low threshold.[4] Theranostics efforts are centered around treating these more lethal, castrate-resistant prostate cancers. The treatment combines a targeting compound or ligand with a radioactive particle which is injected into the patient and targets the cancer cells.
Because it is highly expressed in more than 95 percent of prostate cancers, prostate specific membrane antigen (PSMA) is one of the emerging diagnostic and theranostic biomarkers for prostate cancer detection as well as targeted therapies[5] and is a predictive biomarker for prostate cancer.[6] Clinicians monitor treatment-induced metabolic changes to the tumor, which serves to indicate the likelihood of successful response to treatment. Targeting PSMA in theranostics efforts can help impact clinical management decisions and identify patients who may receive the greatest benefit from targeted therapies.
The FDA recently approved a new lutetium-based therapy, referred to as 177Lu-PSMA-617, based on the results of the Phase III VISION clinical trial.[7] The treatment is for adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC), who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy. Many other small molecules and antibodies targeting PSMA have been developed and labeled, such as 177Lu, 161Tb, 131I, 90Y, 67Cu, 47Sc, and are currently being studied in preclinical and clinical studies.[8]
Supporting the growing field of molecular imaging and radiopharmaceuticals
With the long-term success of PET imaging biomarker 18F-FDG (FDG) in oncology, and newly approved therapies such as 177Lu-PSMA-617, many other useful diagnostic and theranostic biomarker discoveries are likely to gain approval for clinical use to support personalized treatments and improved outcomes. As utilisation of molecular imaging technology expands, clinical interest in new radiopharmaceuticals is continuously growing.[9] An important aspect of introducing these new tracers is the ability to produce and distribute them so clinicians have access to them.
As a leader in the molecular imaging and radiopharmaceutical industry, GE Healthcare supports the continued discovery and production of new tracers and therapies with powerful tools to streamline their production.
Cyclotrons, PET radiochemistry systems and tracer production facility solutions are required to deliver FDG to a large number of clients or supply a research program with a wide range of tracers. Having this support facilitates the efficiency required to work within clinical schedules, the flexibility needed for research protocols and the performance necessary to meet distribution demands.
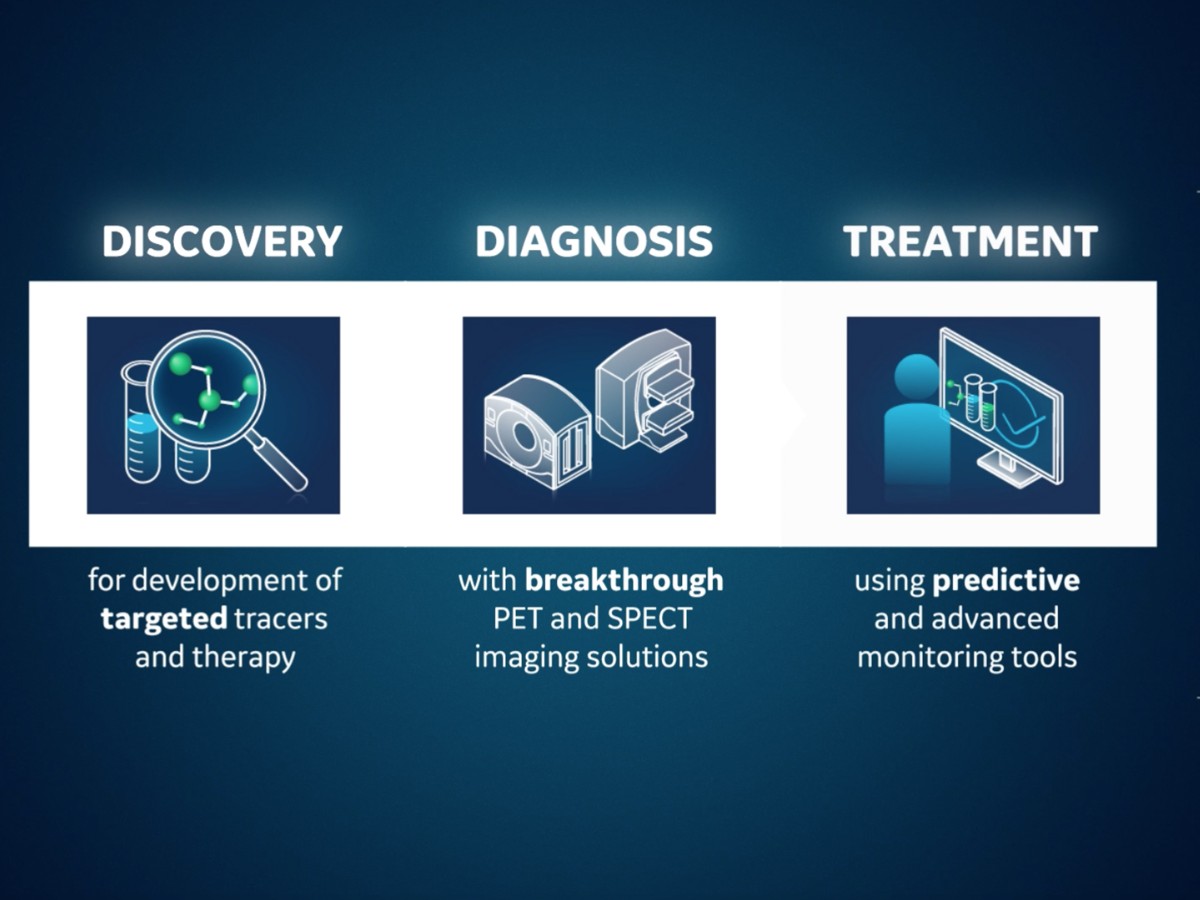
Connecting every step in molecular imaging, from discovery to treatment.
The reliance on molecular imaging techniques such as PET/CT and SPECT/CT to provide clinicians quantitative data about metabolic tumor characterisation, combined with knowledgeable clinicians to acquire, interpret and monitor treatment effectiveness are the keys to the future of and potential for theranostics to become standard of care. PET imaging, for example, can interrogate the whole body for the expression of therapeutic targets.[10] The presence and degree of target expression are associated with a therapy response. Thus, PET imaging probes have been introduced as predictive biomarkers.[11]
Advances in molecular imaging hardware and software continue to facilitate improvements in clinician workflow as well as improvements in exam duration and facilitating interpretation of studies. The introduction of AI and deep learning-based tools also assist with image classification. Experts believe, however, that due to the complexities of molecular medicine, more training is critically important and urgently needed to integrate these approaches into patient management and maintain an optimistic outlook for the growth of the specialty and opportunities in molecular diagnostics and treatment.[12]
Bright future for theranostics and precision medicine
Theranostics is an attractive and quickly developing therapy option for a variety of cancers, such as lymphoma, melanoma, neuroendocrine tumor and prostate cancer. Industry partners such as GE Healthcare continue to support clinicians’ search for new biomarkers and therapies with data rich, high quality diagnostic imaging and image processing tools for tumor characterization and evaluating therapy response. Theranostics approaches, as they are transitioned to standard of care and more widely accessible, have the potential to successfully improve the management and outcomes of patients affected by many cancers, as well as future possible applications in other clinical areas.
RELATED CONTENT
Learn more about GE Healthcare’s molecular imaging offerings, here.
DISCLAIMER
Not all products or features are available in all geographies. Check with your local GE Healthcare representative for availability in your country.
REFERENCES
[1] https://jnm.snmjournals.org/content/61/Supplement_2/263S
[2] Artificial intelligence at the intersection of pathology and radiology in prostate cancer. Diagnostic Interventional Radiology Journal http://www.dirjournal.org/sayilar/103/buyuk/183-188.pdf Accessed 6/13/2019
[3] Leslie SW, Soon-Sutton TL, Sajjad H, et al. Prostate Cancer. [Updated 2022 Feb 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470550/
[4] https://cancer.ca/en/cancer-information/cancer-types/prostate/treatment/castration-resistant-prostate-cancer#:~:text=Castration%2Dresistant%20prostate%20cancer%20(CRPC,or%20hormone%2Dresistant%20prostate%20cancer.
[5] Rahbar A.B., Afshar-Oromieh A., Jadvar H., Ahmadzadehfar H. PSMA Theranostics: Current Status and Future Directions. Mol. Imaging. 2018;17:1536012118776068. doi: 10.1177/1536012118776068.
[6] Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81-85.
[7] https://news.ohsu.edu/2022/03/29/ohsu-researchers-instrumental-in-studying-newly-fda-approved-treatment-for-a-form-of-prostate-cancer#:~:text=The%20FDA%20recently%20approved%20a,Phase%20III%20VISION%20clinical%20trial.
[8] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7918517/#
[9] https://jnm.snmjournals.org/content/52/Supplement_2/36S
[10] Reubi JC, Maecke HR. Approaches to multireceptor targeting: hybrid radioligands, radioligand cocktails, and sequential radioligand applications. J Nucl Med. 2017;58(suppl):10S–16S.
[11] https://jnm.snmjournals.org/content/60/Supplement_2/3S
[12] https://jnm.snmjournals.org/content/60/Supplement_2/3S